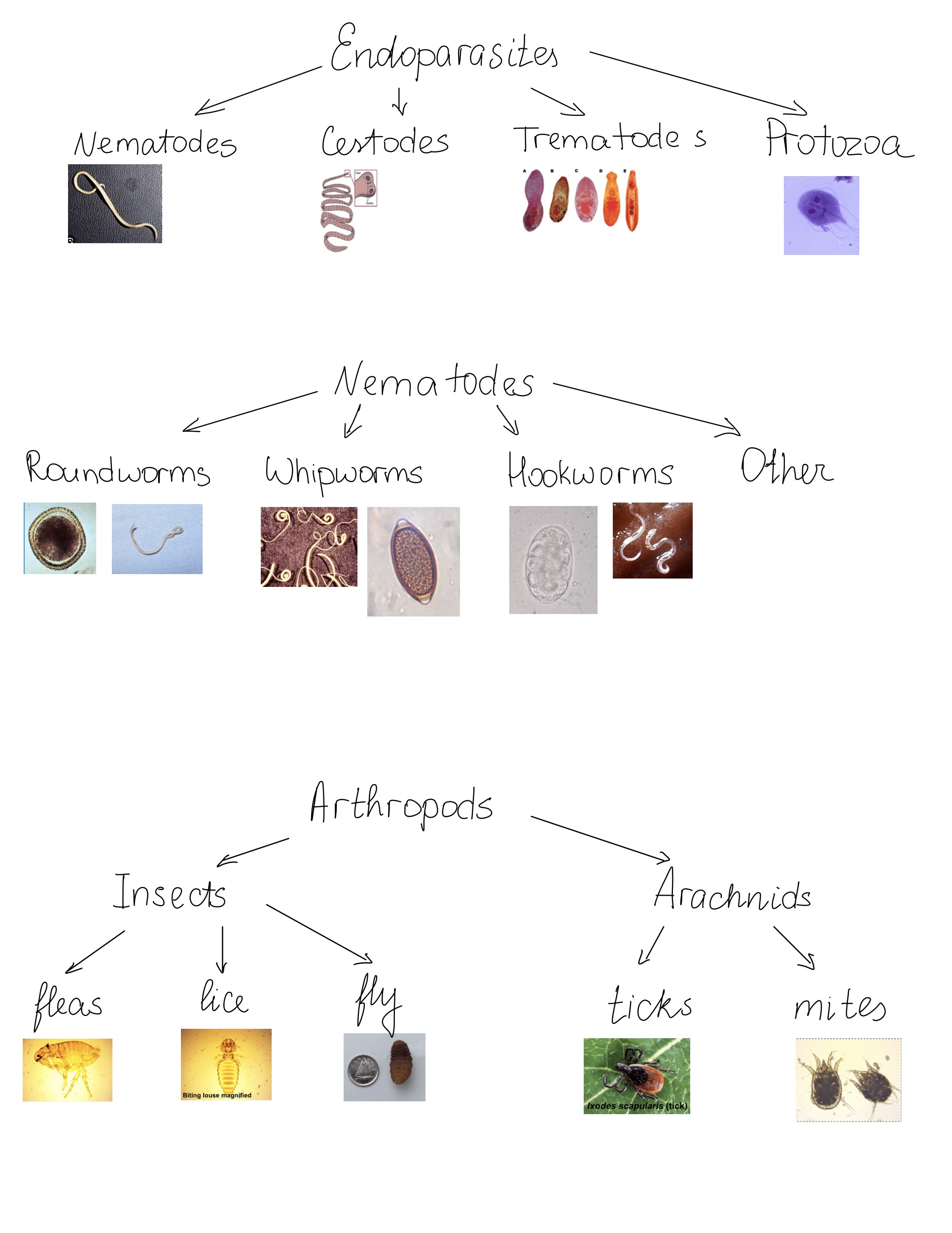
Materials:
- Glass slide
- Glass coverslips
- Wooden applicator stick
- Paper cup
- Waxed paper
- Gauze squares of metal screen tea strainer
- Funnel
- Conical tube
- Sugar flotation solution
- Scale
Procedure:
- Place approx. 5 g of feces in the paper cup.
- Add 30 ml of flotation solution.
-
With the applicator stick, mix the feces to an evenly
suspended emulsion.
-
Using the strainer, pour the liquid into the tube enough to
create a meniscus (dome).
- Place a coverslip on top of the tube.
- Leave the coverslip for 15 minutes.
-
Pick the coverslip and place it on the glass slide with the
fluid down.
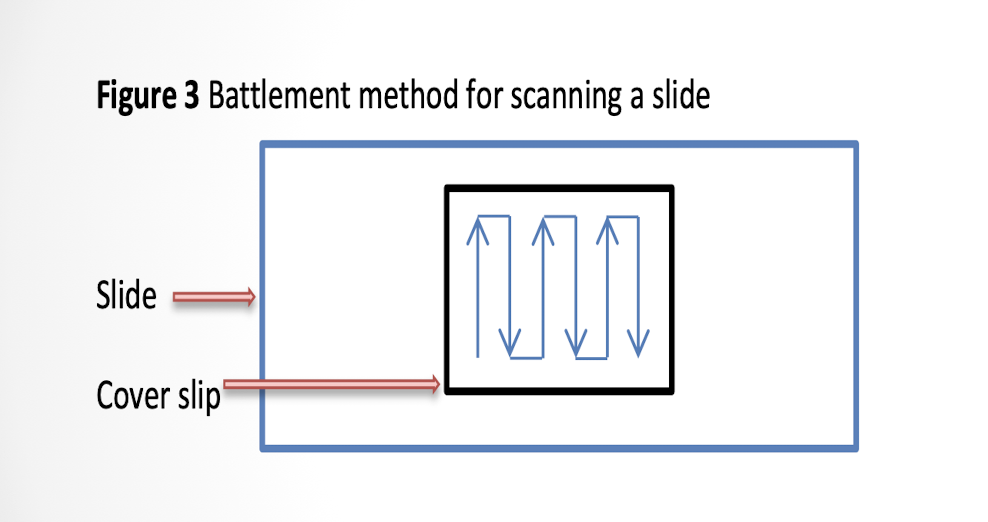
- Examine the slide.
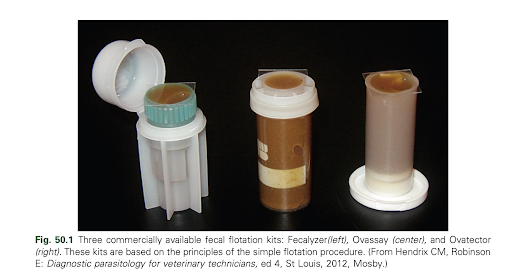
Source - Hickie, J., & Blanchard, L. (2024). Fig. 50.1.
Laboratory Procedures for Veterinary Technicians (7th ed., p.
323). Elsevier Canada.